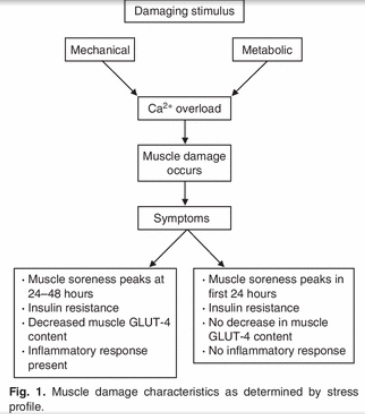
By Dr. Deepak Hiwale. Leave it to the social media PhDs to pontificate on and on about the superiority of resistance training for weight loss. I recently saw a pyramid that touted sleep management over doing actual exercise (i.e., cardio) as better for you regarding fat loss. Now that’s a first. Thus, we know resistance training is superior to aerobic training (aka cardio, endurance training, etc). And most fitness experts seem to agree. But is it really?
Delve a bit deeper into science and you realise that evidence for RT being a better weight-loss tool, is not all that strong and AET (and in some individuals, high-intensity, interval training – HIIT) may be better! Interesting to note here that while RT may have its own set of metabolic benefits, AET may still be better than RT at reducing risks of metabolic disorders too.
Why are you told that resistance training will cause weight loss?
It is a common (and, somewhat dogmatic) belief amongst exercisers, exercise-fitness professionals and clinicians that resistance or strength training (ST), in addition to improving your lean body mass (LBM), is the best way to burn more calories and therefore, lose weight as well.
And, this is how – they’ll tell ya – it (apparently) works:
- RT or ST has the potential to increase your LBM, also called fat-free mass (FFM), including muscle mass – there is enough evidence to support this 1–4
- Skeletal muscle is the most metabolically active tissue in the body – well, no! Its more complicated than that (see below)
- Increase in muscle mass translates into more calories burned throughout the day, even when resting – evidence equivocal (see below)
- Therefore, more muscle you carry, more is your resting metabolic rate (RMR) and more calories you burn throughout the day (increase in total daily energy expenditure (TDEE)
- Increase in TDEE (with or without a nutritional calorific deficit) leads to weight loss
What’s wrong with what they tell you?
While all that sounds good in theory, everything isn’t as cut and dry as they make it out to be:
- Skeletal muscle isn’t the most metabolically active of the tissues in the body – heart and the kidneys are! These organs have the highest metabolic rates, 2x those of the liver and the brain and a whopping 35x that of the skeletal muscle! 5Having said that though, of all the tissues, skeletal muscle may indeed contribute significantly towards energy expended during the day. This is so because skeletal muscle wins on account of sheer mass – it weighs much more than all these other organs mentioned.

- Increased muscle mass does not bump up your metabolism to the point that it will burn additional calories which will translate into weight loss:
- Previous studies examining the effects of RT on RMR have reported mixed results – both in men and women 2,3,6–15
- Only older men (and not older women or younger men and women) show an elevated RMR in response to RT; most studies support this finding 2,3,6,10,11,13
- In younger men & women and in older women, there seems to be a consistent lack of change in RMR in response to RT; the association between RT and rise in RMR all but disappears 7,8,12,14,15
- Recent studies have shown mixed results too – with some showing an increase in RMR in response to RT; 16,17others, a no change. 18,19 Interestingly, one study showed a fall in RMR in response to ‘dieting’, which could not be stopped by resistance training 20
- A rare study that compared the effects of RT on RMR across various age groups, reported no changes in RMR in either young or older individuals! 12
- A study by Lemmer et al. 17reported some curious findings:
- RMR in response to RT is more affected by gender than age; men are more likely to benefit from RT than women
- When younger and older men were pooled together, a significant increase in RMR with RT was shown
- Younger and older women showed no effect on RMR in response to RT
In a nutshell, RT does not alter energy expenditure significantly outside of the exercise session and especially in younger men or in women across all age groups.
Weight / fat loss with resistance training
Misinterpretation of current ACSM and other guidelines 21–23 have led to the dogmatic belief amongst exercise-fitness professionals that RT has conclusively been proven to reduce body weight. In reality, a closer look at existing literature suggests that the evidence for RT as an effective tool for weight-loss remains equivocal, at best. 24–29
- The ACSM guidelines on ‘strategies for weight loss and prevention of weight regain in adults’ states that, ‘research evidence does not support RT as effective for weight loss’ and points
 out that ‘the effects of RT for prevention of weight gain (after initial weight loss) are largely unknown’ 21
out that ‘the effects of RT for prevention of weight gain (after initial weight loss) are largely unknown’ 21 - While few studies have observed some reduction in body fat with RT,30–32others have found no effect on body fat % even when the intervention was continued for 12-52 weeks 33–35
- Interestingly, one study found a gender-based differential effect of RT on body fat – reduction in body fat was observed in the group containing younger and older men pooled together but not in women. 17This finding is not dissimilar to the findings from other studies that RT enhances RMR only in older men 7,8,12,14,15
There is, however, a need to mention here that although RT does not seem to contribute significantly to calorie expenditure outside of the exercise session or fat loss, it is associated with numerous health benefits – increased lean mass, improved work capacity and decreased chronic disease risk factors (sarcopenia), to name a few. 36,37
High-intensity, Interval Training
HIIT, they will tell you, will not only burn calories during the workout but also increase your calorie expenditure through the rest of the day (through increased excess post-exercise oxygen consumption – EPOC – a fancy term the whole town and his wife seems to be using these days!). And, that will translate into weight loss!
EPOC or oxygen debt, as it used to be called previously, is the mechanism by which the body makes up for the oxygen deficit created during an exercise session by increasing oxygen consumption well after cessation of exercise – breathlessness you experience for a few minutes after you’ve climbed to the top of the stairs is an example.
In reality, increase in EPOC after an HIIT session is modest (only 6-15% of total energy expenditure). EPOC alone, therefore, may be insignificant for causing weight loss. 38
Having said that, a study published in 2002 in the European Journal of Applied Physiology utilising circuit type of resistance training with relatively heavy weights and short rest periods generated EPOC which increased resting metabolic rate by 21% and 19% for 24 and 48 hours post- workout. As the authors content, if these numbers are applied to a typical 180-pound individual, it would amount to 773 calories expended over 2 days after cessation of the exercise session! 39 So, HIIT does seem to have benefits.
However, whereas in overweight-obese / untrained individuals, it is difficult to achieve the high-intensity and the duration required to elicit a high enough EPOC to be of any consequence for weight loss. And, prescription of such complex methods of training – needing highly skilled  movements – is likely to reduce exercise enjoyment and long-term adherence in novice and out-of-shape individuals, in seasoned exercisers, HIIT and EPOC may be an effective way to bump up calorie burning and improve body composition.
movements – is likely to reduce exercise enjoyment and long-term adherence in novice and out-of-shape individuals, in seasoned exercisers, HIIT and EPOC may be an effective way to bump up calorie burning and improve body composition.
Aerobic endurance training
Also called ‘low-intensity, steady state’ (LISS) cardio or ‘long, slow distance’ (LSD) training, aerobic endurance training (AET) may just be the best tool out there, for most people when it comes to losing weight.
Researchers from the University of Pittsburgh, Pennsylvania, conducted a study comparing RT with AET in young women 40. The results will come as a surprise (for most)! Apparently, not only is AET better than RT at reducing body fat % but it also wins hands down when it comes to:
- improving cardiorespiratory fitness
- improving insulin sensitivity
- reducing visceral adipose tissue (fat surrounding organs)
- reducing abdominal fat, and
- reducing inter-muscular (within muscle) fat
Other studies have also supported the idea that AET may be better at reducing visceral and  abdominal fat, not to mention, the overall body fat%.
abdominal fat, not to mention, the overall body fat%.
- A study published in Dec, 2012 reported that while AET and combined AET/RT exercise programs caused more weight loss than RT alone, AET/RT and RT resulted in increased lean mass. However, although requiring a double time commitment over AET alone, a combined AET/RT exercise program did not result in ‘significantly more weight loss over AET alone’ 41
- Another study published in the American Journal of Physiology – Endocrinology and Metabolism concluded that AET caused significant reductions in:
- Whole body fat including subcutaneous abdominal fat, visceral adipose tissue (VAT – fat around the organs) and liver fat content
- plasma liver enzymes, esp. alanine aminotransferase (enzyme reflecting the amount of liver damage), and
- HOMA (Homeostasis Model Assessment – a measure of the level of your steady state pancreatic beta cell function (%B) and insulin sensitivity (%S)
Resistance training, on the other hand, failed to significantly affect these variable 42
- Owing to results like these, it shouldn’t come as a surprise that AET is recommended to be central to exercise programs for reducing VAT and its metabolic adverse effects – obesity and other metabolic disorders 43
- Even in the absence of significant weight loss, AET may improve metabolic disease parameters, esp. in patients of type 2 diabetes 44
Women and aerobic endurance training
Why do women prefer conventional AET?
As if the results of the studies mentioned above didn’t come as shocking enough for you, here’s something that is even more thought-provoking – something that might answer your question of why women tend to favour treadmills over free-weights!
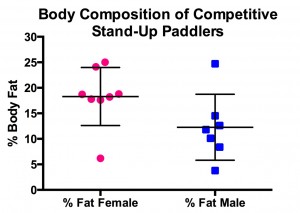
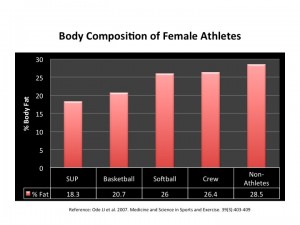
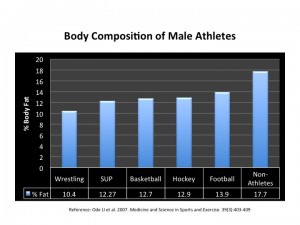
It appears that AET is more effective in (overweight and obese, both young and older) women than in men 40. Furthermore, there is some evidence to suggest that women enjoy AET more than RT 45; the opposite seems to be true with young men – they seem to enjoy RT more (now  come on, do we even need any proof of that?!).
come on, do we even need any proof of that?!).
My hunch is that is that women find AET more enjoyable because it is more effective for them! Not surprisingly then – call it nature or subconscious minds at work – there seems to be a very valid reason why you see more women heading to the treadmill rather than the ‘free-weights section’!
RT or AET and Metabolic Disease
Abdominal obesity is a prominent risk factor for metabolic disease (type II diabetes, cardiovascular disease, etc.). 46 Results from the STRRIDE study suggest that AET was associated with significant reductions in VAT, a measure of abdominal obesity. 47,48
Although in comparison to AET, RT does not cause much difference in measures of fat tissue, it does cause a significant reduction in CRP (a parameter, high levels of which, suggests a low-grade, chronic systemic inflammation with the potential to develop into cardiovascular disease and diabetes type II). 49 Important to note here that an inverse association seems to exist between aerobic fitness and chronic systemic inflammation.50,51 Sedentariness increases inflammatory markers. 49
Conflicting data exists over the superiority of AET over RT for the reduction of metabolic disease risk parameters (HbA1c, blood lipids including triglycerides and LDL particle size). Having said, regular and long-term, moderate intensity exercise seems to increase HDL and lower triglycerides, even in the absence of weight loss. 52
Although RT has benefits of its own, a combination of AET and RT exercise regimen – although more effective at reducing the risk of metabolic disease than RT alone – were not significantly different from AET alone 53. This effectively suggests that the RT component may be contributing precious little (if at all) to the disease prevention effect of an AET-RT exercise program.
A NEAT solution to the problem
Of all the components of human daily energy expenditure (BMR, thermic energy of food, exercise-related activity thermogenesis (EAT) and non-exercise activity thermogenesis (NEAT)), NEAT is the most modifiable parameter and is capable of significantly pushing up your total daily energy expenditure (TDEE) than exercise sessions (!), even in intense exercisers 54. Even very low-level physical activities like mastication (chewing) and fidgeting can increase energy  expenditure by 20-40% above your resting metabolic rate! NEAT includes energy expenditure of walking, talking, going for your job, sitting, toe-tapping, shopping, dancing, etc. It should be apparent that this component (i.e., NEAT) has zero resemblance to resistance training.
expenditure by 20-40% above your resting metabolic rate! NEAT includes energy expenditure of walking, talking, going for your job, sitting, toe-tapping, shopping, dancing, etc. It should be apparent that this component (i.e., NEAT) has zero resemblance to resistance training.
Comment
It is likely that AET (treadmill runs) may be more effective than RT – especially in overweight women – for reducing body fat and preventing metabolic diseases. Also,
- RT seems to contribute very little to weight-loss
- RT doesn’t seem to contribute towards (metabolic) disease prevention-management as much as AET does
- Combination of RT and AET does not seem to afford any more benefits over AET alone when weight loss or metabolic disease management is the prime goal
Conclusion
Looking at much of the evidence, the question that begs to be answered is: ‘what if we were all wrong about our weight-loss exercise strategies and indeed, about our obsession with the fat-burning abilities of resistance training? And, what if those women on treadmills were right all along?!
I reckon, it’s time we stopped ridiculing (or even downright laughing at) those men / women who hit the treadmill every single time they’re at the gym.
Take home message
- Resistance training may be contributing precious little towards calorie burning outside of exercise sessions and eventual weight loss! Furthermore, gains in RMR subsequent to gains in lean body mass are miniscule.
- HIIT in overweight – obese and untrained individuals HIIT may not be ideal; in seasoned exercisers, may lead to significant calorie expenditure both in and outside of the exercise sessions
- Aerobic Endurance training seems to be the best tool for total body weight and fat reduction – needs to a be an integral part of almost every weight-loss program
- Aerobic Endurance training wins hands down for metabolic disease management
- Women do not seem to respond as well to resistance training, aerobic endurance training and HIIT may be better options
- NEAT can contribute significantly to total daily energy expenditure – staying active through the day can really bump your calorific expenditure (probably more so than RT or AET)
About the Author: Facebook link: https://www.facebook.com/pg/drdeepakhiwale/about/?ref=page_internal
‘Conditioning Clinic’ is a brain child of Dr Deepak S Hiwale. Better known internationally as ‘The Fitness Doc, Dr Hiwale prefers and recommends a preventive approach to deal with metabolic diseases. He specializes in disease reversal – obesity, diabetes, cardiovascular diseases, you name it! He is also a strength and conditioning consultant and currently has club and elite cricketers as his clients!
References
- Byrne HK, Wilmore JH. The relationship of mode and intensity of training on resting metabolic rate in women. Int J Sport Nutr Exerc Metab. 2001;11(1):1-14. doi:10.1017/CBO9781107415324.004.
- Campbell WW, Crim MC, Young VR, Evans WJ. Increased energy requirements and changes in body composition with resistance training in older adults. Am J Clin Nutr. 1994;60(2):167-175. http://www.ncbi.nlm.nih.gov/pubmed/8030593. Accessed October 12, 2016.
- Poehlman ET, Toth MJ, Ades PA, Calles-Escandon J. Gender differences in resting metabolic rate and noradrenaline kinetics in older individuals. Eur J Clin Invest. 1997;27(1):23-28. http://www.ncbi.nlm.nih.gov/pubmed/9041373. Accessed October 11, 2016.
- Washburn RA, Donnelly JE, Smith BK, Sullivan DK, Marquis J, Herrmann SD. Resistance training volume, energy balance and weight management: rationale and design of a 9 month trial. Contemp Clin Trials. 2012;33(4):749-758. doi:10.1016/j.cct.2012.03.002.
- Wang Z, Ying Z, Bosy-Westphal A, et al. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am J Clin Nutr. 2010;92(6):1369-1377. doi:10.3945/ajcn.2010.29885.
- Ballor DL, Harvey-Berino JR, Ades PA, Cryan J, Calles-Escandon J. Contrasting effects of resistance and aerobic training on body composition and metabolism after diet-induced weight loss. Metabolism. 1996;45(2):179-183. http://www.ncbi.nlm.nih.gov/pubmed/8596486. Accessed October 12, 2016.
- Broeder CE, Burrhus KA, Svanevik LS, Wilmore JH. The effects of either high-intensity resistance or endurance training on resting metabolic rate. Am J Clin Nutr. 1992;55(4):802-810. http://www.ncbi.nlm.nih.gov/pubmed/1550062. Accessed October 12, 2016.
- Cullinen K, Caldwell M. Weight training increases fat-free mass and strength in untrained young women. J Am Diet Assoc. 1998;98(4):414-418. doi:10.1016/S0002-8223(98)00094-7.
- Pratley R, Nicklas B, Rubin M, et al. Strength training increases resting metabolic rate and norepinephrine levels in healthy 50- to 65-yr-old men. J Appl Physiol. 1994;76(1):133-137. http://www.ncbi.nlm.nih.gov/pubmed/8175496. Accessed October 12, 2016.
- Ryan AS, Pratley RE, Elahi D, Goldberg AP. Resistive training increases fat-free mass and maintains RMR despite weight loss in postmenopausal women. J Appl Physiol. 1995;79(3):818-823. http://www.ncbi.nlm.nih.gov/pubmed/8567523. Accessed October 12, 2016.
- Taaffe DR, Pruitt L, Reim J, Butterfield G, Marcus R. Effect of sustained resistance training on basal metabolic rate in older women. J Am Geriatr Soc. 1995;43(5):465-471. http://www.ncbi.nlm.nih.gov/pubmed/7730525. Accessed October 12, 2016.
- Rall LC, Meydani SN, Kehayias JJ, Dawson-Hughes B, Roubenoff R. The effect of progressive resistance training in rheumatoid arthritis. Increased strength without changes in energy balance or body composition. Arthritis Rheum. 1996;39(3):415-426. http://www.ncbi.nlm.nih.gov/pubmed/8607890. Accessed October 12, 2016.
- Treuth MS, Hunter GR, Weinsier RL, Kell SH. Energy expenditure and substrate utilization in older women after strength training: 24-h calorimeter results. J Appl Physiol. 1995;78(6):2140-2146. http://www.ncbi.nlm.nih.gov/pubmed/7665410. Accessed October 12, 2016.
- Van Etten LM, Westerterp KR, Verstappen FT. Effect of weight-training on energy expenditure and substrate utilization during sleep. Med Sci Sports Exerc. 1995;27(2):188-193. http://www.ncbi.nlm.nih.gov/pubmed/7723641. Accessed October 12, 2016.
- Van Etten LM, Westerterp KR, Verstappen FT, Boon BJ, Saris WH. Effect of an 18-wk weight-training program on energy expenditure and physical activity. J Appl Physiol. 1997;82(1):298-304. http://www.ncbi.nlm.nih.gov/pubmed/9029230. Accessed October 12, 2016.
- Kirk EP, Donnelly JE, Smith BK, et al. Minimal resistance training improves daily energy expenditure and fat oxidation. Med Sci Sports Exerc. 2009;41(5):1122-1129. doi:10.1249/MSS.0b013e318193c64e.
- Lemmer JT, Ivey FM, Ryan AS, et al. Effect of strength training on resting metabolic rate and physical activity: age and gender comparisons. Med Sci Sports Exerc. 2001;33(4):532-541. http://www.ncbi.nlm.nih.gov/pubmed/11283427. Accessed October 11, 2016.
- Hunter GR, Byrne NM, Sirikul B, et al. Resistance training conserves fat-free mass and resting energy expenditure following weight loss. Obesity (Silver Spring). 2008;16(5):1045-1051. doi:10.1038/oby.2008.38.
- Meckling KA, Sherfey R. Randomized Trial of Hypocaloric, High-Protein Diet on Body Compo, Resting Metabolic Rate – meckling2007.pdf. Vol 32.; 2007:743-752. doi:10.1139/H07-059.
- Geliebter A, Maher MM, Gerace L, Gutin B, Heymsfield SB, Hashim SA. Effects of strength or aerobic training on body composition, resting metabolic rate, and peak oxygen consumption in obese dieting subjects. Am J Clin Nutr. 1997;66(3):557-563. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9280173.
- Donnelly JE, Blair SN, Jakicic JM, et al. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459-471. doi:10.1249/MSS.0b013e3181949333.
- Pollock ML, Franklin BA, Balady GJ, et al. Resistance Exercise in Individuals With and Without Cardiovascular Disease. Circulation. 2000;101(7).
- Williams MA, Haskell WL, Ades PA, et al. Resistance Exercise in Individuals With and Without Cardiovascular Disease: 2007 Update. Circulation. 2007;116(5).
- Aldana SG, Greenlaw RL, Diehl HA, et al. Effects of an intensive diet and physical activity modification program on the health risks of adults. J Am Diet Assoc. 2005;105(3):371-381. doi:10.1016/j.jada.2004.12.007.
- Andersen RE, Wadden TA, Bartlett SJ, Zemel B, Verde TJ, Franckowiak SC. Effects of lifestyle activity vs structured aerobic exercise in obese women: a randomized trial. JAMA. 1999;281(4):335-340. http://www.ncbi.nlm.nih.gov/pubmed/9929086. Accessed October 11, 2016.
- Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296-2304. doi:10.1001/jama.298.19.2296.
- Curioni CC, Lourenço PM. Long-term weight loss after diet and exercise: a systematic review. Int J Obes (Lond). 2005;29(10):1168-1174. doi:10.1038/sj.ijo.0803015.
- Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992;56(2):320-328. http://www.ncbi.nlm.nih.gov/pubmed/1386186. Accessed October 11, 2016.
- Delecluse C, Colman V, Roelants M, et al. Exercise programs for older men: mode and intensity to induce the highest possible health-related benefits. Prev Med (Baltim). 2004;39(4):823-833. doi:10.1016/j.ypmed.2004.03.023.
- Ferrara CM, Goldberg AP, Ortmeyer HK, Ryan AS. Effects of aerobic and resistive exercise training on glucose disposal and skeletal muscle metabolism in older men. J Gerontol A Biol Sci Med Sci. 2006;61(5):480-487. http://www.ncbi.nlm.nih.gov/pubmed/16720745. Accessed October 11, 2016.
- Olson TP, Dengel DR, Leon AS, Schmitz KH. Changes in inflammatory biomarkers following one-year of moderate resistance training in overweight women. Int J Obes (Lond). 2007;31(6):996-1003. doi:10.1038/sj.ijo.0803534.
- Polak J, Moro C, Klimcakova E, et al. Dynamic strength training improves insulin sensitivity and functional balance between adrenergic alpha 2A and beta pathways in subcutaneous adipose tissue of obese subjects. Diabetologia. 2005;48(12):2631-2640. doi:10.1007/s00125-005-0003-8.
- Hunter GR, Wetzstein CJ, Fields DA, Brown A, Bamman MM. Resistance training increases total energy expenditure and free-living physical activity in older adults. J Appl Physiol. 2000;89(3):977-984. http://www.ncbi.nlm.nih.gov/pubmed/10956341. Accessed October 11, 2016.
- Hunter GR, Bryan DR, Wetzstein CJ, Zuckerman PA, Bamman MM. Resistance training and intra-abdominal adipose tissue in older men and women. Med Sci Sports Exerc. 2002;34(6):1023-1028. http://www.ncbi.nlm.nih.gov/pubmed/12048332. Accessed October 11, 2016.
- Schmitz KH, Jensen MD, Kugler KC, Jeffery RW, Leon AS. Strength training for obesity prevention in midlife women. Int J Obes Relat Metab Disord. 2003;27(3):326-333. doi:10.1038/sj.ijo.0802198.
- Frontera WR, Meredith CN, O’Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988;64(3):1038-1044. http://www.ncbi.nlm.nih.gov/pubmed/3366726. Accessed October 11, 2016.
- Hurley BF, Redmond RA, Pratley RE, Treuth MS, Rogers MA, Goldberg AP. Effects of strength training on muscle hypertrophy and muscle cell disruption in older men. Int J Sports Med. 1995;16(6):378-384. doi:10.1055/s-2007-973024.
- LaForgia J, Withers RT, Gore CJ. Effects of exercise intensity and duration on the excess post-exercise oxygen consumption. J Sports Sci. 2006;24(12):1247-1264. doi:10.1080/02640410600552064.
- Schuenke M, Mikat R, McBride J. Effect of an acute period of resistance exercise on excess post-exercise oxygen consumption: implications for body mass management. Eur J Appl Physiol. 2002;86(5):411-417. doi:10.1007/s00421-001-0568-y.
- Lee S, Deldin AR, White D, et al. Aerobic exercise but not resistance exercise reduces intrahepatic lipid content and visceral fat and improves insulin sensitivity in obese adolescent girls: a randomized controlled trial. Am J Physiol Endocrinol Metab. 2013;305(10):E1222-9. doi:10.1152/ajpendo.00285.2013.
- Willis LH, Slentz CA, Bateman LA, et al. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J Appl Physiol. 2012;113(12):1831-1837. doi:10.1152/japplphysiol.01370.2011.
- Slentz CA, Bateman LA, Willis LH, et al. Effects of aerobic vs. resistance training on visceral and liver fat stores, liver enzymes, and insulin resistance by HOMA in overweight adults from STRRIDE AT/RT. Am J Physiol Endocrinol Metab. 2011;301(5):E1033-9. doi:10.1152/ajpendo.00291.2011.
- Ismail I, Keating SE, Baker MK, Johnson NA. A systematic review and meta-analysis of the effect of aerobic vs. resistance exercise training on visceral fat. Obes Rev. 2012;13(1):68-91. doi:10.1111/j.1467-789X.2011.00931.x.
- Kadoglou NPE, Iliadis F, Angelopoulou N, et al. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur J Cardiovasc Prev Rehabil. 2007;14(6):837-843. doi:10.1097/HJR.0b013e3282efaf50.
- Lee S, Bacha F, Hannon T, Kuk JL, Boesch C, Arslanian S. Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: a randomized, controlled trial. Diabetes. 2012;61(11):2787-2795. doi:10.2337/db12-0214.
- Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469-480. doi:10.1111/j.1464-5491.2006.01858.x.
- Strasser B. Physical activity in obesity and metabolic syndrome. Ann N Y Acad Sci. 2013;1281:141-159. doi:10.1111/j.1749-6632.2012.06785.x.
- Slentz CA, Aiken LB, Houmard JA, et al. Inactivity, exercise, and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol. 2005;99(4).
- Donges CE, Duffield R, Drinkwater EJ. Effects of resistance or aerobic exercise training on interleukin-6, C-reactive protein, and body composition. Med Sci Sports Exerc. 2010;42(2):304-313. doi:10.1249/MSS.0b013e3181b117ca.
- Aronson D, Sheikh-Ahmad M, Avizohar O, et al. C-Reactive protein is inversely related to physical fitness in middle-aged subjects. Atherosclerosis. 2004;176(1):173-179. doi:10.1016/j.atherosclerosis.2004.04.025.
- Panagiotakos DB, Pitsavos C, Chrysohoou C, Kavouras S, Stefanadis C, ATTICA Study. The associations between leisure-time physical activity and inflammatory and coagulation markers related to cardiovascular disease: the ATTICA Study. Prev Med (Baltim). 2005;40(4):432-437. doi:10.1016/j.ypmed.2004.07.010.
- Carroll S, Dudfield M. What is the relationship between exercise and metabolic abnormalities? A review of the metabolic syndrome. Sports Med. 2004;34(6):371-418. http://www.ncbi.nlm.nih.gov/pubmed/15157122. Accessed October 18, 2016.
- Bateman LA, Slentz CA, Willis LH, et al. Comparison of aerobic versus resistance exercise training effects on metabolic syndrome (from the Studies of a Targeted Risk Reduction Intervention Through Defined Exercise – STRRIDE-AT/RT). Am J Cardiol. 2011;108(6):838-844. doi:10.1016/j.amjcard.2011.04.037.
- Levine JA. NEAT – Levine.pdf. Vol 62.; 2004:S82-97. http://www.ncbi.nlm.nih.gov/pubmed/15387473. Accessed October 16, 2016.











 actuality, each study you do will likely lead to a different experimental question which in turn leads to another investigation. The mark of an excellent scientist isn’t just the ‘answers’ they arrive at, but the questions they generate. I actually enjoy writing. It’s like the icing on the cake. Writing for scientific journals is its own unique skill. There are some individuals
actuality, each study you do will likely lead to a different experimental question which in turn leads to another investigation. The mark of an excellent scientist isn’t just the ‘answers’ they arrive at, but the questions they generate. I actually enjoy writing. It’s like the icing on the cake. Writing for scientific journals is its own unique skill. There are some individuals















 tion (ISSN) and is currently pursuing his MSc. His researc
tion (ISSN) and is currently pursuing his MSc. His researc




 s #1, I’d suggest brushing your teeth and using Scope. If you prefer ‘medicine-like’ breath, Listerine works quite fine. I guess the author would probably tell you to stay away from garlic and onions too. And the author makes the cardinal error of associating high protein diets with low carb consumption. There is absolutely no reason why the two even have to go
s #1, I’d suggest brushing your teeth and using Scope. If you prefer ‘medicine-like’ breath, Listerine works quite fine. I guess the author would probably tell you to stay away from garlic and onions too. And the author makes the cardinal error of associating high protein diets with low carb consumption. There is absolutely no reason why the two even have to go