By Sérgio Fontinhas. Each muscle fiber is a single, multinucleated cell, made up of smaller units called myofibrils. Myofibrils have a repeating pattern called a sarcomere, which is the basic functional unit of muscles. The myofibril is made up of even smaller structures called myofilaments, which are long chains of proteins actin and myosin. Another set of proteins regulate the interaction between actin and myosin. In the actin thin filament there’s a binding site that a myosin head can reach and grab, but the binding sites are covered. Calcium causes configurational changes and uncovers the binding sites for myosin. This calcium is stored in muscle cells in the sarcoplasmic reticulum distributed around the myofibrils. Strength training can result in localized muscle tissue damage. When a certain threshold is exceeded, sarcomeres break. (It is assumed that optimum sarcomere length is 2.5 μm). This damages contractile elements or myofibrillar structures, disrupts the sarcolemma and sarcoplasmic reticular, causes damage to supportive connective tissue and injuries in the cytoskeleton (1). This damage may generate a hypertrophic response (2, 3). Muscle damage is a frequent response after unaccustomed exercise, or when performing high intensity exercise. A trainee may experience stiffness and delayed-onset muscle soreness (aka DOMS). Other metabolic consequences are increases in creatine kinase, muscle troponin I, myoglobin and heavy myosin chain (4).
Eccentric actions – The best way to induce micro tears is by emphasizing eccentric training. The eccentric contraction has been proven through countless studies to cause the most damage, which has been shown to mediate a hypertrophic response (5,6), causing myofibrillar remodeling (7,8). The distribution of sarcomeres on each myofibril is nonuniform, the weakest sarcomeres are located at different regions. This nonuniform lengthening causes a shearing of myofibrils and deforms membranes(4). The presence of disrupted sarcomeres in myofibrils and damage to the excitation–contraction (E–C) coupling system are signs of damage in a muscle from eccentric exercise (9). During active stretch of a muscle, most of the length change will be taken up by the weakest sarcomeres (10) in myofibrils (the weakest half-sarcomeres). These sarcomeres become progressively weaker and then lengthen rapidly, uncontrollably, to a point of no myofilament overlap. Then overstretched sarcomeres are distributed at random along muscle fibers. When the muscle relaxes, some myofilaments in the majority of overstretched sarcomeres become disrupted (11). During repeated eccentric contractions it is postulated that the number of disrupted sarcomeres grows, until a point is reached where membrane damage occurs. It is at this point that damage to elements of the E–C coupling machinery becomes apparent. Subsequently the fiber may die (Fig. 1).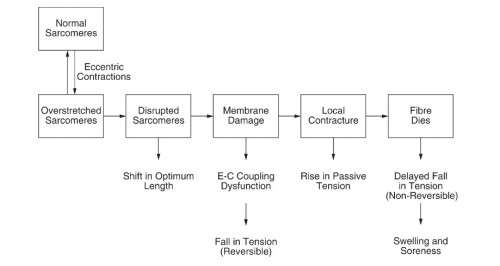
(12) Membrane damage begins with tearing of t-tubules followed by damage to the sarcoplasmic reticulum, uncontrolled Ca2+ release. If the damage is extensive enough, parts of the fiber, or the whole fiber, may die. Breakdown products of dead and dying cells would lead to a local inflammatory response associated with tissue oedema and soreness (12). Although it’s not clear, the first step in the damage process can be t-tubule rupture, leading to inactivation of some sarcomeres, but the reverse sequence beginning with sarcomere disruptions can also lead to t-tubule damage (12). There are also observations of abnormal t-tubular arrangements after eccentric exercise (13).
Eccentric actions and force generation – Muscles achieve higher absolute forces when contracting eccentrically (14–16). Heavy negatives, or supramaximal eccentric actions involve eccentric contractions at a weight greater than concentric 1RM. It has been shown that eccentric strength is approximately 20–50% greater than the concentric strength (17) and even predicted to be up to 64% greater (52). Eccentric contractions could stimulate greater adaptations (18), because increases strength are thought to be proportional to the magnitude of force developed (19). Eccentric training is more effective at increasing total and eccentric strength than concentric training, and appears to be more effective at increasing muscle mass than concentric training, possibly because of the higher forces developed. Adaptations after eccentric training are highly specific to the velocity and type of contraction (20). Eccentric exercise preferentially recruit fast twitch muscle fibers (53,21,22,23) and perhaps recruitment of previously inactive MUs (21,24). This results in an increased mechanical tension in type II fibers, which have the greatest potential for muscle (53,25,26,27). Compared with concentric contractions, eccentric contractions also produce less fatigue and are more efficient at metabolic level. Unaccustomed eccentric contractions produce transient muscle damage, soreness and force impairments.
Eccentric actions and protein synthesis – Passive muscular tension develops because of lengthening of extra myofibrillar elements, especially collagen (28). This increases the active tension enhancing the hypertrophic response. Eccentric contractions elicits greater gains in lean muscle compared with concentric and isometric contractions (29,30,31,32). Maximal muscle hypertrophy can only be attained if eccentric muscle actions are performed (33). When lifting the same weight concentrically and eccentrically no significant difference between the two contractions is observed, if volume is equalized. However in a few studies there’s was a slight advantage for the concentric actions (34,54). Eccentric actions are best done supramaximal, above 1RM concentric load. Lengthening the muscle increase protein synthesis more than a concentric contraction (34), in part by releasing phosphatidic acid, which encourages protein synthesis (35). Another pathway is through the activation of satellite cells located on the outside of muscles. Satellite cells move to the damaged area and fuse to muscle, becoming a part of it (36), increasing muscle fiber size by the addition of the satellite cell’s nucleus to the muscle. The more nuclei, the greater the growth potential. Plateau happens when we can’t adequately activate satellite cells (37,38), therefore maximize eccentric loading may be very beneficial.
Other increases have also been observed, such as a faster rise in protein synthesis (39), greater increases in IGF-1 messenger RNA (mRNA) expression (40), and more pronounced elevations in p70S6k (41), when compared with other types of contractions.
As for the tempo, faster speed eccentric contractions release more growth factors, more satellite cells, and greater protein synthesis than slow speed eccentric contractions (42,43). A 2- to 3-second tempo is hypothesized to be ideal for maximizing a hypertrophic response (43). Eccentric training is also associated with an increased metabolic stress. Higher eccentric intensities elevate lactate buildup and spike anabolic hormonal levels (44).
Muscle swelling and soreness – In human subjects, the initial fall in tension after eccentric exercise is followed by a slow rise over 2–4 h, presumably recovery from metabolic exhaustion. Then 24 h later there’s a second fall in tension (45). Eccentric exercise is followed by sensations of stiffness and soreness the next day (46), transient muscle damage, soreness and force impairments (47). Because of eccentric exercise the contracting muscle is forcibly lengthened. Delayed muscle soreness sets in at about 6–8 h after the exercise and peaks at about 48 h (45,48). A second bout of eccentric exercise, a week after the first, leaves us much less stiff and sore. The injury triggers a local inflammatory response that is accompanied by some oedema. The breakdown products of injured tissues sensitize nociceptors (12,45,49). These nociceptors respond to stimuli that are normally non-noxious, leaving the muscle tender to local palpation, stretch and contraction. A component of the delayed soreness from eccentric exercise may involves large-fiber mechanoreceptors (50,51). The repair mechanism involves the addition of sarcomeres to regenerating muscle fiber, as shown by animal experiments. Neutrophils migrate to the area of micro trauma. Damaged fibers release several agents that attract macrophages and lymphocytes to the injury site. The purpose of macrophages is to remove cellular debris and to produce cytokines that activate myoblasts, macrophages and lymphocytes. This response triggers the release of various growth factors that regulate satellite cell proliferation and differentiation (44).
Stretch overload, hypertrophy and hyperplasia – Hypertrophy involves the enlargement of contractile elements (55). Hyperplasia on the other hand results in an increase in the number of fibers. Increasing muscular density is very painful. However if significant damage is inflicted the number of fibers can actually increase. As noted, eccentric actions cause the most damage, but there’s another method such as stretch overload.
Stretch induced overload is when a certain load stretches a muscle, either intermittently, progressively, or chronically. These methods typically produce more sarcomeres in series (elongation). It’s also strongly associated with hyperplasia (56,57,58,59). This is usually studied in animal models.
1. Intermittent stretch – The intermittent stretch consists of stretching the muscle with the same weight. Stretch periods lasted for 24h in animal models, with 2 or 3 days or rest days in between for recovery. This method produce muscle fiber hypertrophy without fiber hyperplasia (60). Part of the mass increase is due to increases in muscle length. In one study muscle length increased 26.1% in intermittently stretched muscle (60).
2. Progressive stretch overload – Progressive stretch overload of skeletal muscle results in hypertrophy before hyperplasia (61,62). In progressive overload the load is increased every workout, with 2 to 3 days of rest days in between for recovery (61). Again, stretch periods of 24h each. The adaptive response to progressive stretch overload involve an initial fiber hypertrophy – that is increase and peak in cross sectional area and length – followed by hyperplasia (61). Muscle fibers may attain a critical size before the onset of fiber hyperplasia. If fibers enlarge to a critical size and are subjected to further stress they undergo a splitting process, the parent fiber gives rise to two or more daughter fibers (61). This method produced 142% increase in cross-sectional area (in 16 days of stretch muscles); 50% increase in muscle length; 318% increase in muscle mass (after 28 days of stretch). All of these results exceed any reported in the literature.
3. Chronic stretch – Chronic stretch overload results in hyperplasia before hypertrophy (63). Chronic stretch does not allow for a recovery or rest interval and therefore results in significant muscle fiber injury (60,64). Hence, the initial fiber hyperplasia in the chronically stretched model may be an injury-related phenomenon (60).
Training strategies
Intensity – Intensity refers to the load lifted, the closer the load is to 1RM the greater the intensity. Higher intensity is related with low repetition due to the high percentage of load. It’s argue to be the most important variable for growth (44,66), at least to target the fast-twitch fibers. Intensity is customarily expressed as a percentage of 1RM and equates to the number of repetitions that can be performed with a given weight. For this purposed training at high intensity is suggested, as well as intensity of effort (intention of lifting the weight explosively even though it won’t go fast).
Rest interval – Different rest periods have distinct effects on strength capacity and metabolite buildup, thereby impacting the hypertrophic response (44,67). Long rest intervals afford full recovery of strength, maximizing mechanical tension (at the expense of metabolic stress). Most of the strength capacity is recovered within the first minute (44,68). A rest interval of 90-120s may be optimal for maximizing mechanical stress.
Repetitions – A repetition range of 1-5 is considered to be low, 6-12 is considered moderate, and above 15 is considered high. Low repetitions with long rest intervals maximize mechanical tension, due to the higher load one can lift. Therefore low repetition schemes are suggested to maximize muscle damage.
Techniques
1. Heavy negatives – Heavy negatives (supramaximal loaded eccentric actions) is the performance of eccentric contractions at a weight greater than concentric 1RM. This technique usually requires a spotter to help raise the weight. A muscle is not fully fatigued during concentric training (65), therefore the use of heavy negatives is recommended for an additional hypertrophic stimulus.
2. Assisted Negatives – This technique involves performing regular repetitions while a spotter applies pressure on the negative portion of the rep. Using a load above 1RM isn’t always necessary, the point is doing more total eccentric work than concentric, for any number of reps, for example 70% concentric RM and 90% eccentric.
3. Emphasizing the Negative – Taking 3-5 seconds to lower the weight may allow a lifter to induce a maximum amount of damage. Emphasizing the negative may increase the micro tears in your muscles and release more satellite cells.
4. Forced Negatives – After concentric failure there’s still more work, failure is not achieved eccentrically. After concentric failure a spotter can assist on the positive rep while finishing eccentric reps until failure.
5. Loaded stretches – Putting the muscle at the most stretched position and using a load to stretch. This should be with a moderate weight for at least 30s, perhaps at the end of a set. Several approaches are possible: using one stretch after failure in every set, intraset-stretches (instead of rest interval), descending or ascending. Note that the stretching protocol eliciting more gains was the progressive stretch, in which the weight is increased every workout, but can also be increased every set.
Conclusion
Eccentrics, especially supramaximal eccentric contractions produce:
1. The most muscle damage
2. More protein synthesis
3. More hypertrophy
4. More strength
5. More growth factors
6. More satellite cells
Should be performed above with a load above 1RM, with a tempo between 1-3 seconds, and must be used in moderation (risk of muscle fiber death).
References:
1. Hill, M and Goldspink, G. Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage. J Physiol 549: 409–418, 2003.
2. Izquierdo, M, Ibanez, J, Gonzalez-Badillo, JJ,Hakkinen, K, Ratamess, NA, Kraemer, WJ, French, DN, Eslava, J, Altadill, A,Asiain, X, and Gorostiaga, EM. Differential effects of strength training leading to failure versus not to failure on hormonal responses, strength and muscle power increases. J Appl Physiol 100: 1647–1656, 2006.
3. Evans, WJ. Effects of exercise on senescent muscle. Clin Orthopaed Rel Res 403(Suppl.): S211–S220, 2002.
4. Tee JC, Bosch AN, Lambert MI (2007). Metabolic consequences of exercise induced muscle damage. Sports Med 37: 827-836.
5. Evans WJ. Effects of exercise on senescent muscle. Clin Orthop Relat Res 403(Suppl): S211–S220, 2002.
6. Hill M and Goldspink G. Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage. J Physiol 549:409–418, 2003.
7. Crameri RM, Langberg H, Magnusson P, Jensen CH,Schroder HD, Olesen JL, and Kjaer M. Changes in satellite cells in human skeletal muscle after a single bout of high intensity exercise. J Physiol 558:333–340, 2004.
8. Yu JG and Thornell LE. Desmin and actin alterations in human muscles affected by delayed onset muscle soreness: A high resolution immunocy to chemical study. Histochem Cell Biol 118: 171–179, 2002.
9. Warren,G. L. Ingalls, C.P. Lowe, D.A. Armstrong, R.B. (2001). Excitation-contraction uncoupling: major role in contraction-induced muscle injury. Exercise and Sport Sciences Reviews 29, 82–87.
10. Morgan, D. L. (1990). New insights into the behavior of muscle during active lengthening. Biophysics Journal 57,209–221.
11. Talbot, J. A. & Morgan, D. L. (1996). Quantitative analysis of sarcomere non-uniformities in active muscle following a stretch. Journal of Muscle Research and Cell Motility 17,261–268.
12. U. Proske D. L. Morgan. Muscle damage from eccentric exercise: mechanism, mechanical signs,adaptation and clinical applications. Journal of Physiology (2001),537.2, pp.333–345
13. Takekura H., Fujinami, N., Nishizawa, T., Ogasawara,H. & Kasuga, N. (2001). Eccentric exercise-induced morphological changes in the membrane systems involved in excitation– contraction coupling in rat skeletal muscle. Journal of Physiology 533, 571–583.
14. Crenshaw AG, Karlsson S, Styf J, et al. Knee extension torque and intramuscular pressure of the vastus lateralis muscle during eccentric and concentric activities. Eur J Appl Physiol 1995;70:13–19.
15. Westing SH, Cresswell AG, Thortensson A. Muscle activation during maximal voluntary exercise and concentric knee extension. EurJ Appl Physiol 1991;62:104–8.
16. Westing SH, Seger JY. Eccentric and concentric torque velocity characteristics, torque output comparisons and gravity effect torque corrections for the quadriceps and hamstring muscles in females. Int J Sports Med 1989;10:175–80
17. Bamman MM, Shipp JR, Jiang J, Gower BA, Hunter GR,Goodman A, McLafferty CL, and Urban RJ. Mechanical load increases muscle IGF-1and androgen receptor mRNA concentrations in humans. Am J Physiol Endocrinol Metab 280: E383–E390, 2001
18. Hather BM, Tesch PA, Buchanan P, et al. Influence of eccentric actions on skeletal muscle adaptations to resistance training. Acta Physiol Scand 1991;143:177–85
19. Goldberg AL, Etlinger JD, Goldspink DF, et al. Mechanism of work-induced hypertrophy of skeletal muscle. Med Sci Sports Exerc1975;7:185–98.
20. M Roig, K. O’Brien, G. Kirk, R. Murray, P.McKinnon, B. Shadgan,W. D. Reid. The effects of eccentric versus concentric resistance training on muscle strength and mass in healthy adults: a systematic review with meta-analysis, Br J Sports Med 2009; 43:556–568.
21. Nardone A, Romano, C, and Schieppati M. Selective recruitment of high-threshold human motor units during voluntary isotonic lengthening of active muscles. J Physiol 409: 451–471, 1989.
22. Shepstone TN, Tang JE, Dallaire S, Schuenke MD,Staron RS, and Phillips SM. Short-term high- vs. low-velocity isokinetic lengthening training results in greater hypertrophy of the elbow flexors in young men. J Appl Physiol 98: 1768–1776, 2005.
23. Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, and Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol 88:2097–2106, 2000.
24. McHugh MP, Connolly DA, Eston RG, and Gleim GW. Electromyographic analysis of exercise resulting in symptoms of muscle damage. J Sport Sci 18: 163–172, 2000.
25. Hortoba´ gyi, T, Barrier J, Beard D, Braspennincx J, and Koens J. Greater initial adaptations to submaximal muscle lengthening than maximal shortening. J Appl Physiol 81: 1677–1682, 1996
26. Kosek DJ, Kim JS, Petrella JK, Cross JM, and Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol 101: 531–544,2006.
27. Tesch PA. Skeletal muscle adaptations consequent to long-term heavy resistance exercise. Med Sci Sports Exerc 20(5 Suppl):S132–S134, 1988.
28. Toigo, M and Boutellier, U. New fundamental resistance exercise determinants of molecular and cellular muscle adaptations. Eur J Appl Physiol 97: 643–663, 2006.
29. Farthing JP and Chilibeck PD. The effects of eccentric and concentric training at different velocities on muscle hypertrophy. Eur J Appl Physiol 89: 578–586, 2003.
30. Friedmann B, Kinscherf R, Vorwald S, Muller, H,Kucera K, Borisch S, Richter G, Ba¨ rtsch P, and Billeter R. Muscular adaptations to computer-guided strength training with eccentric overload. Acta Physiol Scand 182: 77–88, 2004.
31. Higbie EJ, Cureton KJ, Warren GL III, and Prior BM. Effects of concentric and eccentric training on muscle strength, cross-sectional area, and neural activation. J Appl Physiol 81: 2173–2181, 1996.
32. Norrbrand L, Fluckey JD, Pozzo M, and Tesch PA.Resistance training using eccentric overload induces early adaptations inskeletal muscle size. Eur J Appl Physiol 102: 271–281, 1989.
33. Hather BM, Tesch PA, Buchanan P, and Dudley GA. Influence of eccentric actions on skeletal-muscle adaptations to resistance training. Acta Physiol Scand 143: 177–185, 1991.
34. Eliasson J, et al. Maximal lengthening contractions increase p70 S6 kinase phosphorylation in human skeletal muscle in the absence of nutritional supply. Am J Physiol Endocrinol Metab. 2006 Dec;291(6):E1197-205.
35. O’Neil TK, et al. The role of phosphoinositide 3-kinase and phosphatidic acid in the regulation of mammalian target of rapamycin following eccentric contractions. J Physiol. 2009 Jul 15;587 (Pt 14):3691-701.
36. Rosenblatt JD, et al. Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle Nerve. 1994Jun;17(6):608-13.
37. Bamman MM, et al. Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. J Appl Physiol (1985).2007 Jun;102(6):2232-9.
38. Petrella JK, et al. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol (1985). 2008 Jun;104(6):1736-42.
39. Moore DR, Phillips SM, Babraj JA, Smith K, and Rennie MJ. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metabol 288: E1153–E1159, 2005.
40. Shepstone TN, Tang JE, Dallaire S, Schuenke MD,Staron RS, and Phillips SM. Short-term high- vs. low-velocity isokinetic lengthening training results in greater hypertrophy of the elbow flexors inyoung men. J Appl Physiol 98: 1768–1776, 2005.
41. Eliasson J, Elfegoun T, Nilsson J, Kohnke R,Ekblom B, and Blomstrand E. Maximal lengthening contractions increase p70 S6kinase phosphorylation in human skeletal muscle in the absence of nutritionalsupply. Am J Physiol Endocrinol Metabol 291:E1197–E1205, 2006.
42. Moore DR, et al. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab. 2005 Jun; 288(6):E1153-9.
43. Shepstone TN, et al. Short-term high- vs. low-velocity isokinetic lengthening training results in greater hypertrophy of the elbow flexors in young men. J Appl Physiol (1985). 2005 May; 98(5):1768-76.
44. Schoenfeld BJ. The mechanisms of muscle hypertrophy and their application to resistance training. J Strength Cond Res24: 2857–2875, 2010.
45. Macintyre, D. L., Reid, W. D. & Mckenzie, D.C. (1995). Delayed muscle soreness. The inflammatory response to muscle injury and its clinical implications. Journal of Sports Medicine 20,24–40.
46. Hough, T. (1902). Ergographic studies in muscular soreness. American Journal of Physiology 7, 76–92.
47. Byrne C, Twist C, Eston R. Neuromuscular function after exercise-induced muscle damage. Sports Med 2004;34:49–69.
48. Jones, C., Allen, T., Talbot, J., Morgan, D. L.& Proske, U. (1997). Changes in the mechanical properties of human and amphibian muscle after eccentric exercise. European Journal of Applied Physiology and Occupational Physiology 76, 21–31.
49. Smith, L. L. (1991). Acute inflammation: the underlying mechanism in delayed onset muscle soreness? Medicine and Science inSports and Exercise 23, 542–551.
50. Barlas, P., Walsh, D. M., Baxter, G. D. & Allen,J. M. (2000). Delayed onset muscle soreness: effect of an ischaemic block upon mechanical allodynia in humans. Pain 87, 221–225.
51. Weerakkody, N. S. Whitehead, N.P. Canny, B. J.Gregory J. E. & Proske. (2001). Large-fiber mechanoreceptors contribute to muscle soreness after eccentric exercise. Journal of Pain 2,209–219.
52. Moir GL, Erny KF, Davis SE, Guers JJ, Witmer CA.The Development of a Repetition-Load Scheme for the Eccentric-Only Bench Press Exercise. J Hum Kinet. 2013 Oct 8;38:23-31.
53. Brad Schoenfeld. The Use of Specialized Training Techniques to Maximize Muscle Hypertrophy. Strength & Conditioning Journal: August 2011 – Volume 33 – Issue 4 – pp 60-65
54. Mayhew TP, et al. Muscular adaptation to concentric and eccentric exercise at equal power levels. Med Sci Sports Exerc.1995 Jun;27(6):868-73.
55. Vierck, J, O’Reilly, B, Hossner, K, Antonio, J,Byrne, K, Bucci, L, and Dodson, M. Satellite cell regulation following myotrauma caused by resistance exercise. Cell Biol Int 24: 263–272, 2000.
56. Tsika R: The Muscular System: The Control of Muscle Mass. In: ACSM’s Advanced Exercise Physiology. Edited by Tipton C. Baltimore, MD: Lippincott Williams& Wilkins; 2006: 161-177.
57. Siu Pm AS: Age-related apoptotic responses to stretch-induced hypertrophy in quail slow-tonic skeletal muscle. American Journal of Cell Physiology 2005, 285(5):C1105-1113.
58. Kelley G: Mechanical overload and skeletal muscle fiber hyperplasia: a meta-analysis. Journal of Applied Physiology 1996, 81:1584-1588.
59. Paul A. RN: Different modes of hypertrophy in skeletal muscle fibers. TheJ ournal of Cell Biology 2002, 156(4):751-760.
60. Jose Antonio, William J. Gonyea. Role of musclefiber hypertrophy and hyperplasia in intermittently stretched avian muscle. J.Appl. Physiol. 7414): 1893-1898, 1993.
61. Jose Antonio, William J. Gonyea. Progressive stretch overload in skeletal muscle results in hypertrophy before hyperplasia.J. Appl. Physiol. 75(3): 1263-1271,
62. Progressive overload of the anterior latissimus dorsi of the adult quail produces muscle fiber hypertrophy before fiber hyperplasia. Physiologist 35: 196, 1992
63. Alway, S. E., W. J. Goneya, M. E. Davis. Muscle fiber formation and fiber hypertrophy during the onset of stretch-overload. Am.J. Physiol. 259 (Cell Physiol. 28): C92-C102, 1990
64. Winchester, P. K., W. J. Goneya. Regional injury and terminal differentiation of satellite cells in stretched avian slow tonic muscle.Deu. Biol. 151: 459-472, 1992
65. Iida, K, Itoh, E,Kim, DS, del Rincon, JP, Coschigano, KT, Kopchick, JJ, and Thorner, MO. Muscle mechano growth factor is preferentially induced by growth hormone in growth hormone deficient lit/lit mice. J Physiol 15; 560: 341–349, 2004.
66. Fry, AC. The role of resistance exercise intensity on muscle fiber adaptations. Sport Med 34: 663–679, 2004.
67. Willardson, JM. A brief review: Factors affecting the length of the rest interval between resistance exercise sets. J Strength Cond Res 20: 978–984, 2006
68. Stull, GA and Clarke, DH. Patterns of recovery following isometric and isotonic strength decrement. Med Sci Sports 3: 135–139, 1971.






