By Sergio Fontinhas – Skeletal muscle hypertrophy refers to an increase in cell size (due primarily to the addition of myofibrillar protein) following chronic heavy resistance training or  tension-induced overload. As a consequence, muscle fibers grow in cross-sectional area and the entire muscle becomes thicker. Several factors regulate this adaptive response, including hormones, genetics and protein synthesis.
tension-induced overload. As a consequence, muscle fibers grow in cross-sectional area and the entire muscle becomes thicker. Several factors regulate this adaptive response, including hormones, genetics and protein synthesis.
Hormones
Hormones as IGF-1, testosterone, and growth hormone play a major role in this response (1,2,3), so when these hormone levels are reduced as in elderly populations the hypertrophic response is blunted (4,5). However it must be noted that acute short-term rises in these hormones after training have a negligible effect on hypertrophy (6,7,8,9,10,11,12,13). It’s only in the case of supraphysiological levels that these hormones make a difference (9).
Skeletal muscle adaptation is an intrinsic process. McMaster University has done much of this work. For example, in one study the same subjects performed both resistance and endurance exercise on each leg and showed different adaptations for each leg. Resistance exercise stimulated both myofibrillar and mitochondrial protein synthesis, while endurance exercise stimulated mitochondrial protein synthesis but not myofibrillar protein synthesis (75).
Exercise programs should not be centered on the manipulation of acute exercise variables and multi-joint exercises seeking to induce a favorable ‘anabolic’ hormonal milieu.
Genetics
Genetics is a key factor in the variability between individuals (6,14,15,110,111), in fact subjects can be stratified as low, moderate and high responders (16,17,112). High responders can have 4 to 5 times greater hypertrophic response compared to low responders, and interestingly some subjects are in fact non-responders and can even lose muscle mass despite proper training and nutrition. In one paper the hypertrophic range in cross-sectional area (CSA) for type I and Type 2 fibers was between -22 to 106% and -4 to 67% respectively (6). In another cluster analysis the range for nonresponders was -16 ±99 µm2 for CSA (17).
This variability is related to changes in microRNA androgen expression (6,18,19), satellite cell number for remodeling (20,21,22,23,24,25,26,27,28), intramuscular anabolic signaling protein activation (29), protein synthesis (30,31), and genetic variation (32,99). In one study investigating the systemic correlates of resistance training-induced hypertrophy (16wk), the change (increase) in androgen receptor protein content and the magnitude of the protein kinase p70S6K phosphorylation (a target of mTOR) after 5h, accounted for 46% of the variance in the hypertrophic response (6). Some of the subjects had a 1.5-2.5 fold increase in AR protein content, suggested to account for about 25% of the variability.
Protein synthesis
The muscle protein synthesis acute response from exercise is a dose-response depending upon exercise intensity and workload. After a latent period after exercise of about 45 minutes to an hour (33), MPS rises sharply (2-3 fold) between 45 and 150 min. This increase in MPS may be sustained for up to 4h in the fasted state after exercise (33), and in the presence of increased AA availability up to 24-48h after exercise (34,35) or even 72 (103) before returning to baseline.
Remarkably, even training after fasting (overnight) the rate myofibrillar protein fractional synthetic rate is still elevated over breakdown (36).  This means that we are not catabolic in the fasted state. The increased synthesis over breakdown appears to come from non-myofibrillar proteins (i.e. collagen, sarcoplasmic, and/or mitochondrial proteins), because muscle protein breakdown is also elevated after exercise (36). The exercise stimulus is therefore the greatest anabolic signal.
This means that we are not catabolic in the fasted state. The increased synthesis over breakdown appears to come from non-myofibrillar proteins (i.e. collagen, sarcoplasmic, and/or mitochondrial proteins), because muscle protein breakdown is also elevated after exercise (36). The exercise stimulus is therefore the greatest anabolic signal.
However, acute measures (1-6h post exercise) of MPS following an initial exposure to RE in novices are not correlated with muscle hypertrophy following chronic resistance training (87). There’ also a review on the relationship between acute of muscle protein synthesis response and changes in muscle mass (88). Muscle protein breakdown is also important for the regulation of muscle hypertrophy on the long term, and the chronic (positive) balance between MPS and MPB is more important than considering acute rises in MPS.
There are many ways and mechanisms of hypertrophy, as summarized by Schoenfeld (81): mechanical tension, muscle damage and metabolic stress. There is no “one-size fits all”, and some will simply respond better or worse than others. Despite all this interindividual variability, there are some general evidence-based recommendations for hypertrophy, regarding exercise programs.
Load and Repetition Range
Mechanical tension seems to be the primary drive for the hypertrophic response (37,38). Mechanical forces are converted into chemical signals in a process called mechanotransduction. This causes molecular and cellular responses in myofibers and satellite cells (24), and mechanical stress alone can directly stimulate mTOR (initiation of protein synthesis) (39,40).
A muscle does not know what it contracts against; it just contracts or relaxes (85).
Training to failure and recruiting as many motor units as possible seems optimal (41). This intensity of effort (training to failure) is perhaps the single most influential controllable variable for enhancing muscular strength, however there’s a diminished ability of untrained subjects to recruit motor units limits (42). The recruitment of motor units and muscle fibers stimulates muscular growth irrespective of what has caused that recruitment. This can be achieved with higher or lower loads and respectively lower or higher repetitions (42). Lighter loads lifted to the point of failure result in a similar amount of muscle fiber activation compared with heavier loads, and both fiber types are stimulated to a roughly equivalent extent (44,45).
There appears to be no difference in the hypertrophic response so long as fatigue is induced. Lifting heavy or lighter loads, there’s roughly equivalent hypertrophy and strength gains (43,44,45,46,47,48,49,50,101).
– One study compared 80%RM vs 30%RM sets to failure with no significant differences between groups for “recreationally active subjects” (44);
– Another compared 3 sets with 75%RM to 4 sets with 30%RM to “volitional fatigue”, again with similar increases in muscle cross-sectional area for untrained subjects (45);
– 3–5 vs. 20–28 of repetitions for each exercise, “until fatigue” with approximately equal volume, also showed no differences for “physically active” subjects (46);
– lower loads, when combined with vascular occlusion, promote equivalent hypertrophy than heavier loads with the same number of sets and similar volume: 50%-30%RM vs. 80%-50%RM “until failure” for “relatively well-trained subjects” (48) and 50%RM vs. 80%RM “to exhaustion” in untrained subjects (49);
-In another study by Schoenfeld et al. (50) comparing powerlifting style training (low reps, higher loads) versus hypertrophy style (higher reps and moderate loads), this time with equalized volume and also to momentary muscular failure, there was no difference in the hypertrophy magnitude after 8 weeks for “well-trained men”.
(Note: untrained subjects will respond well to any stimulus, just like obese subjects will respond well to any diet, however note that the same trend is found in trained subjects, otherwise it would be irrelevant.)
However lifting moderate loads for moderate repetitions is less taxing to the nervous system, joints, and is time efficient compared to higher loads and low repetition ranges, subjects from the hypertrophy group could do more volume if necessary (50).
Training to failure could sometimes lead to overuse injuries (51,52) and for some people could even reduce the levels of IGF-1 hormones responsible for muscle growth after at least 11 weeks (53).
So in short, so longer momentary failure is achieved it doesn’t matter how many reps are performed and under what load.
Repetition Duration
Repetition duration appears to have no significant impact on hypertrophy. Repetition duration can vary within a set, but a certain muscular tension threshold is necessary (perhaps above 30%RM) and muscular failure are requirements (42).
Rest Intervals
The evidence also suggests that rest interval appears to play a role in acute performance (repetitions performed and load lifted) but does not affect the chronic strength or hypertrophic gains acquired, if volume is equalized for comparison (42,54).
Volume
One meta-analysis by James Krieger suggests that multiple-sets (3-6) per exercise (per session) is associated with 40% greater hypertrophy-related Effect Size than 1 set, in both trained and untrained subjects (55).  However there is one critique of that meta-analysis (56), with the authors suggesting that only one set per exercise to failure is necessary (42). Another meta-analysis supports the notion that higher-volume, multiple-set protocols is superior over single set protocols for hypertrophy, for untrained subjects, with the difference becoming more evident as progression occurs (57).
However there is one critique of that meta-analysis (56), with the authors suggesting that only one set per exercise to failure is necessary (42). Another meta-analysis supports the notion that higher-volume, multiple-set protocols is superior over single set protocols for hypertrophy, for untrained subjects, with the difference becoming more evident as progression occurs (57).
Contraction Types
There are different types of muscle contractions: the concentric or positive motion; the eccentric or negative; isometric. There is a difference in muscle fiber recruitment and activation in each contraction and thus a different in force production.
Muscles achieve higher absolute forces when contracting eccentrically (58,59,60). Eccentric strength is approximately 20–50% greater than the concentric strength (61), even predicted to be up to 64% greater (62), and stimulates greater adaptations (63) and appears to be more effective at increasing muscle mass than concentric training.
Eccentric exercise preferentially recruits fast twitch muscle fibers (64,65,66,67) and perhaps recruitment of previously inactive MUs (65,68). This results in an increased mechanical tension in type II fibers, which have the greatest potential for muscle growth (64,69,70,71). A single bout of eccentric exercise results also in a greater increase in IGF-I mRNA expression than a single bout of concentric exercise (72).
Heavy negatives, assisted negatives, or supramaximal eccentric actions with a weight greater than concentric 1RM are some techniques that can applied for this goal. Since a muscle is not fully fatigued during concentric training (73), the use of heavy negatives is recommended. There’s also the use of a flywheel or isokinetic equipment to overload the ECC phase (74), but in this case the contractions can be below the concentric 1RM, but at the end of the set there’s more total volume/load for the concentric actions.
Isometric contractions consists in holding a static contraction, the muscle doesn’t shorten or lengthen. Isometric muscle actions can also induce hypertrophy (76, 77) and should be included in a training program.
Contraction speed
Faster concentric repetitions (1s vs. 3s) are more beneficial for hypertrophy (78). Faster/heavier eccentric repetitions leads to greater hypertrophy in type II fibers, and strength gains than slower/lighter eccentric repetitions (79). Faster speed eccentric contractions release more growth factors, more satellite cells, and greater protein synthesis than slow speed eccentric contractions (80,81). A 2-3 second tempo is hypothesized to be ideal for maximizing a hypertrophic response (80).
Very slow velocities (i.e., superslow training) is suboptimal for strength and hypertrophy (81,82,83). There are some proponents of superslow training, and some studies have been published in non-scientific journals. For example a study by Westcott (84) claimed superslow to be slighter superior for strength than traditional training (for elderly individuals), although the results were not statistically significant, and it wasn’t peer-reviewed.
The majority of the literature indicates superslow training to be suboptimal for general populations. Special populations may benefit from this, injured individuals or elderly suffering from osteoporosis, as it was developed for anyway.
Range of Motion (ROM), “muscle shape” and Non-Uniform Muscle Growth
Full ROM is associated with significant greater strength and hypertrophy gains than a shorter ROM. One study found for full ROM a greater increase in strength (18% vs. 4%) and a greater increase in hypertrophy (60 vs. 15%) at  the distal cross-sectional area closest to the joints (knee or elbow), or insertions (86). The average hypertrophy across the full length of the muscle belly was more than double for the full ROM (44% vs. 21%). Also muscle fiber pennation angles (fiber directions) increased more with full ROM (11% vs. 7% but not statistically significant).
the distal cross-sectional area closest to the joints (knee or elbow), or insertions (86). The average hypertrophy across the full length of the muscle belly was more than double for the full ROM (44% vs. 21%). Also muscle fiber pennation angles (fiber directions) increased more with full ROM (11% vs. 7% but not statistically significant).
Another study also showed significant difference for site specific CSA in favor of the full ROM after 12wk (89). However shorter ROM can in some instances still produce significant hypertrophy to the same extent as full ROM (90), persons with injuries or diminished ROM may benefit from this.
Skeletal muscle fibers rarely just span from origin to insertion. Jose Antonio did a review on the Non-Uniform Muscle Growth and regional adaptation in skeletal muscle (91). Skeletal muscle is a heterogeneous tissue that exhibits numerous inter- and intramuscular differences: architecture, fiber composition, and muscle function (91).
With different exercises selective recruitment of different regions of a muscle can be achieved, so that there’s no single exercise that can maximize the hypertrophic response of all regions of a particular muscle (91). Several muscles are compartmentalized so that fibers terminate intrafascicularly (within the fascicle) and each subdivision is in turn innervated by its own nerve branch with different motor unit territories.
A few examples:
Schoenfeld et al (92) investigated muscle activation for two hamstrings exercises: the stiff leg deadlift and the lying leg curl. Activation of the upper hamstrings was similar between exercises, but the activation of the lower hamstrings, both medially and laterally, was significantly greater in the lying leg curl (170% and 65% respectively).
In one study, researchers confirmed regional differences in muscle hypertrophy (MRI). This corresponded to regional differences in muscle activation (EMG) in the triceps (93). The area of the triceps with the most muscle activation had more hypertrophy after 12 weeks. Furthermore, there was greater hypertrophy on the distal (versus proximal) section of the triceps muscle (93).
The same authors in other study correlated muscle activation (MR) for elbow extensors after one training session for one group with the hypertrophy from another group performing 12 weeks of training. Significantly lower activation in the distal region which was correlated with significantly less hypertrophy in the distal region compared with other areas (100).
For maximal hypertrophy of an entire muscle various exercises must be executed to purportedly stimulate growth in a regional- specific manner. In other words, exercise selection and variety is necessary.
Detraining
Generally hypertrophy becomes evident after around 3-4 weeks of resistance training (94,95,96). Detraining periods have also been considered. One study examined training and detraining in 4 subjects during 100 days, roughly the same rate of atrophy was observed during the detraining phase (40 days) as for the hypertrophy rate during the training phase (60 days) (97).
Another study examined subjects across age and gender groups using the same relative training stimulus (98). After 9 weeks of training muscle volume was twice as much for men (across ages) as for the women (across ages), but after 31 weeks of detraining men also experienced the greater loss of muscle mass; and muscle volume for women returned to original pre-training muscle size only for the females.
However other studies have shown a lesser degree of atrophy or no significant atrophy at all (101,102). A detraining phase of 3 weeks appears to have not much of a difference in muscle mass and adaptations (101). In another similar study by the same authors, while one group trained continuously for 24 weeks the other group performed three cycles of 6-week training (or retraining), with 3-week detraining periods between training cycles; improvements in muscle CSA and strength were similar between the groups (102).
A detraining phase of 3 weeks appears to have not much of a difference in muscle mass and adaptations (101). In another similar study by the same authors, while one group trained continuously for 24 weeks the other group performed three cycles of 6-week training (or retraining), with 3-week detraining periods between training cycles; improvements in muscle CSA and strength were similar between the groups (102).
(In both studies the rate of increase in muscle CSA and 1-RM decreased gradually after 6 week for the control group, while for both studies the experimental group increase in muscle CSA and strength was better, suggesting a more efficient response after a detraining phase.)
However detraining is not the same as deloading, or taper phase.
Concurrent Resistance
Resistance training combined with aerobic exercise in a single program is known as concurrent training (104). Wilson et al. did meta-analysis of 21 studies with a total of 422 effect sizes. Concurrent training results in decrements in strength, hypertrophy and power (although overall power is the major variable affected), however while some individuals experience strength decrements others experience substantial gains. The interference effect may be a result of overreaching and overtraining and stimulates competing adaptations over a long-term training program. The longer the endurance activity the greater the interference.
Specific signals imposed by variations in the duration, modality, and type of exercise are recognized by muscle tissue (75). The adaptations for each modality are vastly different and in most cases conflict one another, and are primarily body part specific. Endurance exercise preferentially increases net protein synthesis in the mitochondrial subfraction (75,107) whereas resistance training preferentially increases net protein synthesis in the myofibrillar subfraction (75,104).
Another example, endurance exercise can decrease the speed of contraction in fast-twitch fibers (5 times faster) and increase the contraction speed in slow-twitch fibers after 10 days of training, interestingly they return to baseline after a detraining period (108).
There’s also fiber conversions, Kraemer et al. observed that concurrent training can cause a conversion from type IIB to type IIA, in terms of percentage; and in terms of area it was found a significant increase in type IIA (109). This data suggests that type I increases, and also type IIA at the expense of type IIB decrease (conversion). In the endurance only group, type IIA and IIC increased in percentage with a decrease in IIB; areas for type I and IIC decreased, resulting in some muscle loss (this group did long duration cardio and also HIIT, HIIT may have played a role in increase in the percentages observed). In the strength group only, the increase in type IIA was greater, at a greater expense of conversion from type IIB. Also all areas for type I, type IIC and Type IIA increased (109).
Endurance exercise before resistance training impairs the upregulation of translation initiation via the PI3K-AKT-mTOR signaling (104,105,106); and inhibits important elongation factors (eef2) responsible for increasing protein synthesis and maintains this inhibition for the duration of the activity (104,45).
In concurrent training, running, but not with cycling, results in significant decrements in both hypertrophy and strength (104), possibly because cycling is more biomechanically similar to the exercises performed for strength and resistance training. Running has also a high eccentric component, as opposed to cycling consisting primarily of concentric actions. Eccentric actions create greater damage, increasing muscle damage in long distance running. Moreover, sprinting (cycling) or HIIT (running) mimics the exercises and intensities often performed for strength and resistance training, and should be used on non-training days, if necessary for some reason.
References:
1. Ahtiainen JP, Pakarinen A, Alen M. Muscle hypertrophy, hormonal adaptations and strength development during strength training in strength-trained and untrained men. Eur J Appl Physiol 2003; 89: 555-63.
2. Velloso CP. Regulation of muscle mass by growth hormone and IGF-1. Br J Pharmacol 2008; 154(3): 557-68.
3. West DWD, Phillips SM. Associations of exercise-induced hormone profiles and gains in strength and hypertrophy in a large cohort after weight training. Eur J Appl Physiol 2012; 112: 2693-702.
4. Hameed M, et al. The effect of recombinant human growth hormone and resistance training on IGF-I mRNA expression in the muscles of elderly men. J Physiol 555: 231–240, 2004.
5. Gruenewald DA, Matsumoto AM. Testosterone supplementation therapy for older men: potential benefits and risks. J Am Geriatr Soc. 2003 Jan;51(1):101-15;
6. Mitchell CJ, Churchward-Venne TA, Bellamy L, Parise G, Baker SK, et al. (2013). Muscular and Systemic Correlates of Resistance Training-Induced Muscle Hypertrophy. PLoS ONE 8(10): e78636. doi:10.1371/journal.pone.0078636
7. West DW, Phillips SM (2012) Associations of exercise-induced hormone profiles and gains in strength and hypertrophy in a large cohort after weight training. Eur J Appl Physiol 112: 2693-2702. doi:10.1007/s00421-011-2246-z. PubMed: 22105707.
8. McKay BR, O’Reilly CE, Phillips SM, Tarnopolsky MA, Parise G (2008) Co-expression of IGF-1 family members with myogenic regulatory factors following acute damaging muscle-lengthening contractions in humans. J Physiol 586: 5549-5560. doi:10.1113/jphysiol.2008.160176.
9. West DW, Phillips SM. Anabolic Processes in Human Skeletal Muscle: Restoring the Identities of Growth Hormone and Testosterone. Phys Sportsmed. 2010 Oct;38(3):97-104.
10. West DW, Burd NA, Staples AW, Phillips SM. Human exercise-mediated skeletal muscle hypertrophy is an intrinsic process. Int J Biochem Cell Biol. 2010 Sep;42(9):1371-5.
11. Wilkinson SB, Tarnopolsky MA, Grant EJ, Correia CE, Phillips SM (2006). Hypertrophy with unilateral resistance exercise occurs without increases in endogenous anabolic hormone concentration. Eur J Appl Physiol 98:546–555
12. West DW, Kujbida GW, Moore DR, Atherton P, Burd NA, Padzik JP, De LM, Tang JE, Parise G, Rennie MJ, Baker SK, Phillips SM (2009) Resistance exercise-induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. J Physiol 587:5239–5247
13. Spiering BA, Kraemer WJ, Anderson JM, Armstrong LE, Nindl BC, Volek JS, Judelson DA, Joseph M, Vingren JL, Hatfield DL, Fragala MS, Ho JY, Maresh CM (2008) Effects of elevated circulating hormones on resistance exercise-induced Akt signaling. Med Sci Sports Exerc 40:1039–1048
14. Hubal MJ, Gordish-Dressman H, Thompson PD, Price TB, Hoffman EP. Variability in muscle size and strength gain after unilateral resistance training. (2005) Med Sci Sports Exerc 37: 964-972.
15. Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, Phillips SM. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr 86: 373–381, 2007.
16. Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol 104: 1736–1742, 2008.
17. Marcas M. Bamman, John K. Petrella, Jeong-su Kim, David L. Mayhew and James M. Cross. Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. J Appl Physiol 102:2232-2239, 2007
18. Davidsen PK, Gallagher IJ, Hartman JW, Tarnopolsky MA, Dela F,Helge JW, Timmons JA, Phillips SM. High responders to resistance exercise training demonstrate differential regulation of skeletal muscle microRNA expression. J Appl Physiol 110: 309–317.
19. Bamman MM, Shipp JR, Jiang J, Gower BA, Hunter GR et al. (2001). Mechanical load increases muscle IGF-I and androgen receptor mRNA concentrations in humans. Am J Physiol Endocrinol Metab 280: E383- E390.
20. Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J ApplPhysiol 104: 1736–1742, 2008.
21. West DWD, Phillips SM. Associations of exercise-induced hormone profiles and gains in strength and hypertrophy in a large cohort after weight training. Eur J Appl Physiol 2012;112: 2693-702.
22. Vierk J, O’Reilly B, Hossner K. Satellite cell regulation following myotrauma caused by resistance exercise. Cell Biol Int 2000; 24: 26372.
23. E., D. L. JARYSZAK, AND C. R. VALLIERE. Response of satellite cells to focal skeletal muscle injury. Muscle Nerve 8: 217-222,1985
24. Toigo M, Boutellier U. New fundamental resistance exercise determinants of molecular and cellular muscle adaptations. Eur J Appl Physiol 97: 643–663, 2006.
25. Zammit PS. All muscle satellite cells are equal, but are some more equal than others? J. Cell. Sci. 121: 2975–2982, 2008
26. Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. Anat. Rec. 170: 421–435, 1971.
27. Bellamy LM, Joanisse S, Grubb A, Mitchell CJ, McKay BR, et al. (2014) The Acute Satellite Cell Response and Skeletal Muscle Hypertrophy following Resistance Training. PLoS ONE 9(10): e109739. doi:10.1371/journal.pone.0109739
28. Kadi, F., Schjerling, P., Andersen, L. L., Charifi, N., Madsen, J. L., Christensen, L. R., & Andersen, J. L. (2004). The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. The Journal of Physiology, 558(Pt 3), 1005–1012. doi:10.1113/jphysiol.2004.065904
29. Terzis G, Georgiadis G, Stratakos G, Vogiatzis I, Kavouras S, Manta P, Mascher H, Blomstrand E. Resistance exercise-induced increase in muscle mass correlates with p70S6 kinase phosphorylation in human subjects. Eur J Appl Physiol 102: 145–152, 2008.
30. Chesley A, MacDougall JD, Tarnopolsky MA, et al. Changes in human muscle protein synthesis after resistance exercise.J Appl Physiol 1992; 73(4): 1383-8.
31. Burd NA, Andrews RJ, West DWD, et al. Muscle time under tension during resistance exercise stimulates differential muscle protein sub-fractional synthetic responses in men. J Physiol 2012; 351-62.
32. Lutoslawska G, Tkaczyk J, Keska A. Myostatin and its role in the regulation of muscle mass and metabolism. Med Sport 2012; 16(4): 165-74.
33. Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N & Rennie MJ (2009). Age-related differences in the dose–response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 587, 211–217
34. Cuthbertson DJ, Babraj J, Smith K, Wilkes E, Fedele MJ, Esser K & Rennie M (2006). Anabolic signaling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol Endocrinol Metab 290, E731–E738.
35. Phillips SM, Tipton KD, Aarsland A,Wolf SE &Wolfe RR (1997). Mixed muscle protein synthesis and breakdown following resistance exercise in humans. Am J Physiol 273, E99–E107.
36. Paul L. Kim1, Robert S. Staron2 and Stuart M. Phillips. Fasted-state skeletal muscle protein synthesis after resistance exercise is altered with training. J Physiol 568.1 (2005) pp 283–290
37. Goldberg AL, Etlinger JD, Goldspink DF, et al. Mechanism of work-induced hypertrophy of skeletal muscle. Med Sci Sports. 1975 Fall;7(3):185–98.
38. Jones, DA and Rutherford, OM. Human muscle strength training: The effects of three different regimens and the nature of the resultant changes. J Physiol 391: 1–11, 1987.
39. Hornberger TA, Chu WK, Mak YW, et al. The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci USA. 2006;103(12):4741–6.
40. Zou K, Meador BM, Johnson B, Huntsman HD, Mahmassani Z, Valero MC, Huey KA, and Boppart MD. The α₇β₁-integrin increases muscle hypertrophy following multiple bouts of eccentric exercise. J Appl Physiol 111: 1134–1141, 2011.
41. Willardson JM. The application of training to failure in periodized multiple-set resistance exercise programs. J Strength Cond Res 21: 628–631, 2007.
42. Fisher J, Steele J, Bruce-Low S, Smith D. Evidence-Based Resistance Training Recommendations. Med Sport 2013;15(3): 147-62.
43. Fuglevand AJ, Zackowski KM, Huey KA, Enoka RM. Impairment of neuromuscular propagation during human fatiguing contractions at submaximal forces. J Physiol 460: 549–572, 1993.
44. Mitchell CJ, Churchward-Venne TA, West DW, Burd NA, Breen L, Baker SK, Phillips SM. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J Appl Physiol 113: 71–77, 2012.
45. Ogasawara R, Loenneke JP, Thiebaud RS, and Abe T. Low-load bench press training to fatigue results in muscle hypertrophy similar to high-load bench press training. International Journal of Clinical Medicine 4: 114–121, 2013.
46. Leger B, Cartoni R, Praz M, Lamon S, Deriaz O, Crettenand A, Gobelet C, Rohmer P, Konzelmann M, Luthi F, Russell AP. Akt signalling through GSK-3beta, mTOR and Foxo1 is involved in human skeletal muscle hypertrophy and atrophy. J Physiol 576: 923–933, 2006.
47. Takarada Y, Sato Y, Ishii N. Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur J Appl Physiol 86: 308–314, 2002.
48. Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol 88: 2097–2106, 2000.
49. Tanimoto M, Ishii N. Effects of low-intensity resistance exercise with slow movement and tonic force generation on muscular function in youngmen. J Appl Physiol 100: 1150–1157, 2006.
50. Schoenfeld BJ, Ratamess NA, Peterson MD, Contreras B, Sonmez GT, Alvar BA. Effects of different volume-equated resistance training loading strategies on muscular adaptations in well-trained men. J Strength Cond Res. 2014 Oct;28(10):2909-18
51. Willardson JM. The application of training to failure in periodized multiple-set resistance exercise programs. J Strength Cond Res. 2007 May;21(2):628-31.
52. Ivan Chulvi Medrano. Muscular failure training in conditioning neuromuscular programs. Journal of Human Sport & Exercise Vol. V No II 2010 19 6-213
53. Mikel Izquierdo , Javier Ibañez , Juan José González-Badillo. Differential effects of strength training leading to failure versus not to failure on hormonal responses, strength, and muscle power gains. Journal of Applied Physiology. 1 May 2006Vol. 100no. 5, 1647-1656DOI
54. Ahtiainen JP, Pakarinen A, Alen M, et al. Short vs. long rest period between the sets in hypertrophic resistance training: influence on muscle strength, size, and hormonal adaptations in trained men. J Strength Cond Res 2005; 19(3): 572-82.
55. Krieger J. Single vs. Multiple sets of resistance exercise for muscle hypertrophy: a meta-analysis. J Strength Cond Res 2010; 24(4): 1150-9.
56. Fisher J. Beware the meta-analysis; is multiple set training really better than single set training for muscular hypertrophy? J Exerc Physiol 2012; 15(6): 23-30
57. Wolfe, BL, LeMura, LM, and Cole, PJ. Quantitative analysis of single- vs. multiple-set programs in resistance training. J Strength Cond Res 18: 35–47, 2004.
58. Crenshaw AG, Karlsson S, Styf J, et al. Knee extension torque and intramuscular pressure of the vastus lateralis muscle during eccentric and concentric activities. Eur J Appl Physiol 1995;70:13–19.
59. Westing SH, Cresswell AG, Thortensson A. Muscle activation during maximal voluntary exercise and concentric knee extension. EurJ Appl Physiol 1991;62:104–8.
60. Westing SH, Seger JY. Eccentric and concentric torque velocity characteristics, torque output comparisons and gravity effect torque corrections for the quadriceps and hamstring muscles in females. Int J Sports Med 1989;10:175–80
61. Bamman MM, Shipp JR, Jiang J, Gower BA, Hunter GR,Goodman A, McLafferty CL, and Urban RJ. Mechanical load increases muscle IGF-1and androgen receptor mRNA concentrations in humans. Am J Physiol Endocrinol Metab 280: E383–E390, 2001
62. Moir GL, Erny KF, Davis SE, Guers JJ, Witmer CA.The Development of a Repetition-Load Scheme for the Eccentric-Only Bench Press Exercise. J Hum Kinet. 2013 Oct 8;38:23-31.
63. Hather BM, Tesch PA, Buchanan P, et al. Influence of eccentric actions on skeletal muscle adaptations to resistance training. Acta Physiol Scand 1991;143:177–85
64. Brad Schoenfeld. The Use of Specialized Training Techniques to Maximize Muscle Hypertrophy. Strength & Conditioning Journal: August 2011 – Volume 33 – Issue 4 – pp 60-65
65. Nardone A, Romano, C, and Schieppati M. Selective recruitment of high-threshold human motor units during voluntary isotonic lengthening of active muscles. J Physiol 409: 451–471, 1989.
66. Shepstone TN, Tang JE, Dallaire S, Schuenke MD,Staron RS, and Phillips SM. Short-term high- vs. low-velocity isokinetic lengthening training results in greater hypertrophy of the elbow flexors in young men. J Appl Physiol 98: 1768–1776, 2005.
67. Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, and Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol 88:2097–2106, 2000.
68. McHugh MP, Connolly DA, Eston RG, and Gleim GW. Electromyographic analysis of exercise resulting in symptoms of muscle damage. J Sport Sci 18: 163–172, 2000.
69. Hortoba´ gyi, T, Barrier J, Beard D, Braspennincx J, and Koens J. Greater initial adaptations to submaximal muscle lengthening than maximal shortening. J Appl Physiol 81: 1677–1682, 1996
70. Kosek DJ, Kim JS, Petrella JK, Cross JM, and Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol 101: 531–544,2006.
71. Tesch PA. Skeletal muscle adaptations consequent to long-term heavy resistance exercise. Med Sci Sports Exerc 20(5 Suppl):S132–S134, 1988.
72. Bamman MM, Shipp JR, Jiang J, Gower BA, Hunter GR, Goodman A, McLafferty CL Jr, and Urban RJ. Mechanical load increases muscle IGF-I and androgen receptor mRNA concentrations in humans. Am J Physiol Endocrinol Metab 280: E383–E390, 2001.
73. Iida, K, Itoh, E,Kim, DS, del Rincon, JP, Coschigano, KT, Kopchick, JJ, and Thorner, MO. Musclemechano growth factor is preferentially induced by growth hormone in growth hormone deficient lit/lit mice. J Physiol 15; 560: 341–349, 2004.
74. Norrbrand L, Fluckey JD, Pozzo M, et al. Resistance training using eccentric overload induces early adaptations in skeletal muscle size. Eur J Appl Physiol 2008; 102: 271-81
75. Sarah B. Wilkinson, Stuart M. Phillips, Philip J. Atherton, Rekha Patel, Kevin E. Yarasheski, Mark A. Tarnopolsky and Michael J. Rennie. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol 586.15 (2008) pp 3701–3717
76. Jones DA, Rutherford OM. Human muscle strength training: the effects of three different regimes and the nature of the resultant changes. J Physiol 1987; 391: 1-11.
77. Davies J, Parker DF, Rutherford OM, et al. Changes in strength and cross sectional area of the elbow flexors as a result of isometric strength training. Eur J Appl Physiol1988; 57: 667-70
78. Nogueira, W, Gentil, P, Mello, SN, Oliveira, RJ, Bezerra, AJ, and Bottaro, M. Effects of power training on muscle thickness of older men. Int J Sport Med 30: 200–204, 2009.
79. Tim N. Shepstone , Jason E. Tang , Stephane Dallaire , Mark D. Schuenke , Robert S. Staron , Stuart M. Phillips. Short-term high- vs. low-velocity isokinetic lengthening training results in greater hypertrophy of the elbow flexors in young men. Journal of Applied Physiology Published 1 May 2005Vol. 98no. 5, 1768-1776
80. Moore DR, et al. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab. 2005 Jun; 288(6):E1153-9.
81. Schoenfeld, BJ. The mechanisms of muscle hypertrophy and their application to resistance training. J Strength Cond Res 24(10): 2857–2872, 2010
82. Keeler, LK, Finkelstein, LH, Miller, W, and Fernhall, B. Early-phase adaptations of traditional-speed vs. SuperSlow resistance training on strength and aerobic capacity in sedentary individuals. J Strength Cond Res 15: 309–314, 2001.
83. Neils, CM, Udermann, BE, Brice, GA, Winchester, JB, and McGuigan, MR. Influence of contraction velocity in untrained individuals over the initial early phase of resistance training. J Strength Cond Res 19: 883–887, 2005.
84. Westcott, W. L., Winett, R. A., Anderson, E. S., Wojcik, J. R., Loud, R. L., Cleggett, E., & Glover, S. (2001). Effects of regular and slow speed resistance training on muscle strength. The Journal of sports medicine and physical fitness, 41(2), 154-158.
85. Fisher J, Steele J, Bruce-Low S, Smith D. Evidence-Based Resistance Training Recommendations. Med Sport 2011; 15(3): 147-62.
86. McMahon GE, Morse CI, Burden A, Winwood K, Onambélé GL. Impact of range of motion during ecologically valid resistance training protocols on muscle size, subcutaneous fat, and strength. J Strength Cond Res. 2014 Jan;28(1):245-5
87. Mitchell CJ, Churchward-Venne TA, Parise G, Bellamy L, Baker SK, et al. (2014) Acute Post-Exercise Myofibrillar Protein Synthesis Is Not Correlated With Resistance Training-Induced Muscle Hypertrophy in Young Men. PLoS ONE 9(2): e89431
88. Cameron J. Mitchell , Tyler A. Churchward-Venne , David Cameron-Smith , Stuart M. Phillips. What is the relationship between the acute muscle protein synthetic response and changes in muscle mass? Journal of Applied PhysiologyPublished 25 September 2014
89. Eugene McMahon G, Onambélé-Pearson G. Impact of range of motion during ecologically valid resistance training protocols, on muscle size, subcutaneous fat and strength. J Strength Cond Res 2013
90. Pinto RS, Gomes N, Radaelli R, et al. Effect of range of motion on muscle strength and thickness. J Strength Cond Res 2012; 26(8): 2140-5.
91. Antonio, J. Nonuniform response of skeletal muscle to heavy resistance training: can bodybuilders induce regional muscle hypertrophy? J. Strength Cond. Res. 14(1):102–113. 2000
92. Schoenfeld BJ, Contreras B, Tiryaki-Sonmez G, Wilson JM, Kolber MJ, Peterson MD. Regional Differences in Muscle Activation During Hamstrings Exercise. J Strength Cond Res. 2014 Jun 24.
93. Wakahara T, Fukutani A, Kawakami Y, Yanai T. Nonuniform muscle hypertrophy: its relation to muscle activation in training session. Med Sci Sports Exerc. 2013 Nov;45(11):2158-65
94. O. R. Seynnes , M. de Boer , M. V. Narici. Skeletal hypertrophy and architectural changes in response to high–intensity resistance training. J Appl Physiol 2007; 102: 368-73.
95. Abe T, DeHoyos DV, Pollock ML. Time course for strength and muscle thickness changes following upper and lower body resistance training in men and women. Eur J Appl Physiol 2000; 81: 174-80.
96. Ogasawara R, Thiebaud RS, Loenneke JP, et al. Time course for arm and chest muscle thickness changes following bench press training. Interventional Med Appl Sci 2012; 4(4): 217-20
97. Narici MV, Roi GS, Landoni. Changes in force, crosssectional area and neural activation during strength training and detraining of the human quadriceps. Eur J Appl Physiol 1989; 59: 310-9.
98. Ivey FM, Roth SM, Ferrell RE, et al. Effects of age, gender, and myostatin genotype on the hypertrophic response to heavy resistance strength training. J Gerontol Med Sci 2000; 55(11): M641-748.
99. Kraemer, WJ, Hakkinen, K, Newton, RU, Nindl, BC, Volek, JS, McCormick, M, Gotshalk, LA,Gordon, SE, Fleck, SJ, Campbell,WW, Putukian, M, and Evans, WJ. Effects of heavy-resistance training on hormonal response patterns in younger vs. older men. J Appl Physiol 87: 982–992, 1999.
100. Taku Wakahara, Naokazu Miyamoto, Norihide Sugisaki, Koichiro Murata, Hiroaki Kanehisa, Yasuo Kawakami, Tetsuo Fukunaga, Toshimasa Yanai. Association between regional differences in muscle activation in one session of resistance exercise and in muscle hypertrophy after resistance training. European Journal of Applied Physiology April 2012, Volume 112, Issue 4, pp 1569-1576
101. Ogasawara R, Yasuda T, Sakamaki M, et al. Effects of periodic and continued resistance training on muscle CSA and strength in previously untrained men. Clin Physiol Funct Imaging 2011; 31: 399-404.
102. Ogasawara R, Yasuda T, Ishii N, et al. Comparison of muscle hypertrophy following 6-month of continuous and periodic strength training. Eur J Appl Physiol 2013; 113: 975-85.
103. Creer, A, Gallagher, P, Slivka, D, Jemiolo, B, Fink, W, and Trappe, S. Influence of muscle glycogen availability on ERK1/2 and Akt signaling after resistance exercise in human skeletal muscle. J Appl Physiol 99: 950–956, 2005.
104. Wilson, JM, Marin, PJ, Rhea, MR, Wilson, SMC, Loenneke, JP, and Anderson, JC. Concurrent training: A meta-analysis examining interference of aerobic and resistance exercise. J Strength Cond Res 26(8): 2293–2307, 2012
105. Creer, A, Gallagher, P, Slivka, D, Jemiolo, B, Fink, W, and Trappe, S. Influence of muscle glycogen availability on ERK1/2 and Akt signaling after resistance exercise in human skeletal muscle. J Appl Physiol 99: 950–956, 2005.
106. Hawley, JA. Molecular responses to strength and endurance training: Are they incompatible? Appl Physiol Nutr Metab 34: 355–361, 2009.
107. MacLean, DA, Graham, TE, and Saltin, B. Branched-chain amino acids augment ammonia metabolism while attenuating protein breakdown during exercise. Am J Physiol 267: E1010–E1022, 1994.
108. R. H. Fitts , D. L. Costill , P. R. Gardetto. Effect of swim exercise training on human muscle fiber function. Journal of Applied Physiology Published 1 January 1989Vol. 66no. 1, 465-475
109. W. J. Kraemer , J. F. Patton , S. E. Gordon , E. A. Harman , M. R. Deschenes, K. Reynolds , R. U. Newton , N. T. Triplett , J. E. Dziados. Compatibility of high intensity strength and endurance training on hormonal and skeletal muscle adaptations. Journal of Applied Physiology Published 1 March 1995 Vol. 78no. 3, 976-989
110. Lutoslawska G, Tkaczyk J, Keska A. Myostatin and its role in the regulation of muscle mass and metabolism. Med Sport 2012; 16(4): 165-74.
111. Stewart CEH, Rittweger J. Adaptive processes in skeletal muscle: Molecular regulators and genetic influences. J Musculoskelet Neuronal Interact 2006; 6(1): 73-86.
112. Hubal MJ, Gordish-Dressman H, Thompson PD, et al. Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc 2005; 37(6): 964-72
 the importance of (whey) protein and all the benefits it brings with it. But now, thanks to those sneaky business and marketing guys (and gals), the process of finding that ‘perfect’ protein is even more challenging than ever. You are probably thinking that the protein you are religiously buying is the best. Maybe it is or maybe it isn’t. I’m not here to promote any company or to criticize what you are doing, I’m simply writing this as an informational tool for you to use when choosing your next batch of protein. As Jose always told me in grad school, “if it helps or has a neutral effect, then do it.”
the importance of (whey) protein and all the benefits it brings with it. But now, thanks to those sneaky business and marketing guys (and gals), the process of finding that ‘perfect’ protein is even more challenging than ever. You are probably thinking that the protein you are religiously buying is the best. Maybe it is or maybe it isn’t. I’m not here to promote any company or to criticize what you are doing, I’m simply writing this as an informational tool for you to use when choosing your next batch of protein. As Jose always told me in grad school, “if it helps or has a neutral effect, then do it.”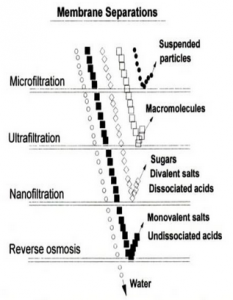 yourself the basic question of what is my intention and purpose is for taking a whey protein supplementation. Going back to the marketing standpoint, you will see adjectives like dynamic, ultra, mega ultra, triple maximal deluxe ultra and others like it just to get your attention. This is a way that Company X claims that their protein is the purest protein on the planet. Just keep in mind that even though a company might have the most elite and elaborate filtration system known to man, they could still add cheap and dangerous fillers to negate the cost of their expensive technique [2]. That’s neither here nor there.
yourself the basic question of what is my intention and purpose is for taking a whey protein supplementation. Going back to the marketing standpoint, you will see adjectives like dynamic, ultra, mega ultra, triple maximal deluxe ultra and others like it just to get your attention. This is a way that Company X claims that their protein is the purest protein on the planet. Just keep in mind that even though a company might have the most elite and elaborate filtration system known to man, they could still add cheap and dangerous fillers to negate the cost of their expensive technique [2]. That’s neither here nor there.